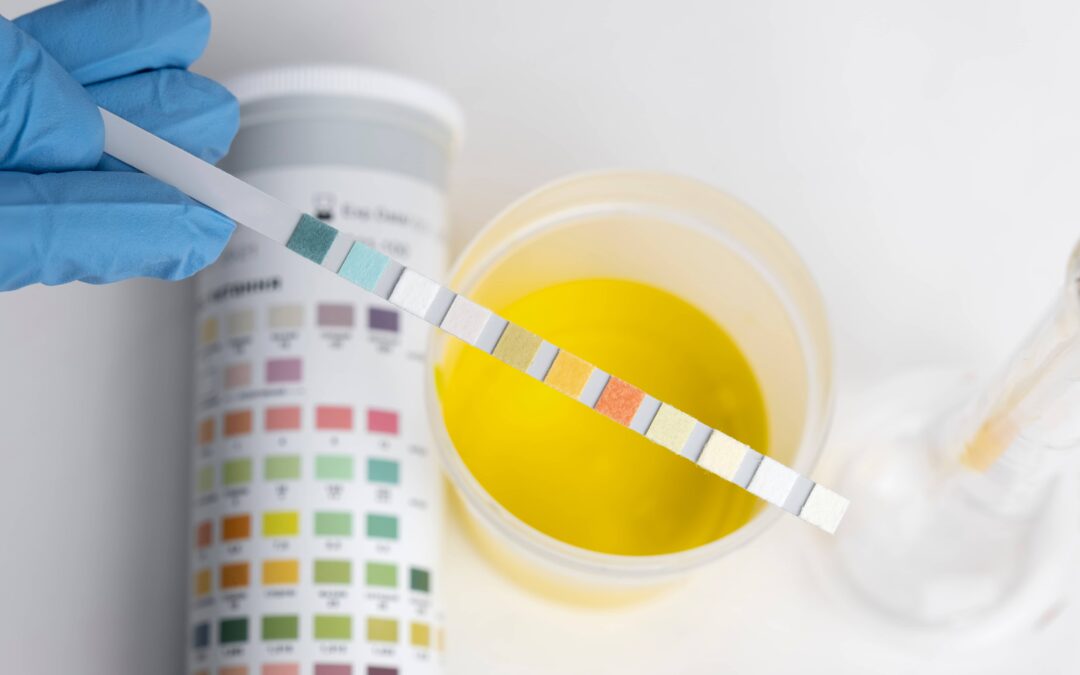
What Is EtG?
The ethyl glucuronide (EtG) is a metabolite of ethanol and is used in EtG alcohol detection. Ethanol is formed when an individual consumes alcoholic beverages. EtG is a byproduct of ethanol and glucuronide. The liver is a source of storage of drugs and alcohol in the human body. It then gets excreted through urine which is the reason why urine is a good source to detect alcohol consumption and provides accurate results in alcohol metabolite testing.
Alcohol can be found in hair, nails, and blood apart from urine. However, urine is the most common way alcohol is tested. Breadth alcohol testing is commonly used in accident cases and immediately after an incident for e.g. a workplace incident.
How Does EtG Testing Work?
EtG is retained in urine for a longer period than breadth or blood. EtG can remain in the human body for as much as 48 hours and if a person is a heavy drinker, it can be present in the urine for up to 72 hours.
Various cut-off levels will indicate the levels of drinking in a person:
- High alcohol consumption (showing high positive outcome) – A person that has had a high alcohol consumption (>1,000 ng/mL) within a given day or 24 hours previously.
- Low alcohol consumption (showing a low positive outcome) – A person who drinks heavily within the last 1 – 3 days or light drinking within the last 24 hours.
How Accurate Is EtG Testing?
Although EtG testing is accurate, there are many products that we are exposed to daily that could cause results to be skewed. Some examples of other alcoholic products include cleaning products, cosmetics, mouthwash, hand sanitisers and other products. This could impact the outcome of a test.
Most lab-based alcohol tests can overcome this as the process can determine the consumption of alcohol in the range of 70% – 85%. EtG tests do not measure the number of drinks a person has had. It is just an indicator of alcohol in a person’s body.
Legal and Ethical Issues Of EtG Testing
Substance use disorders including alcohol disorders (EtG Tests), may have a stigma attached to them. It is critical that the integrity and confidentiality of the patient are kept under strict confidence and privacy. Individuals with the disorder provide information that is personal. This information must be managed to the patient’s benefit i.e. encouraging patients to get into counseling. Much of the testing conducted in laboratories becomes a source of information for organizations to take relevant actions. This information must be transmitted and communicated under the strict chain of custody and develop the patient’s and tester’s trust.
It is important that testers, test for ONLY the requested test I.e. an alcohol test if it is an EtG test. Tests beyond the requested test can produce confidence issues between the patient and the tested. In the spirit of good communication and transparency, the patient will agree and formally authorize the test that he/she will be made to do. This helps build trust between the tested and the patient. The patient will approve the dissemination of the results.
Federal Law
The concern about the adverse effects that social stigma and discrimination have on patients in recovery (and how those adverse effects might deter people from entering treatment) led Congress to pass legislation and the Department of Health and Human Services (DHHS) to issue a set of regulations to protect information about patients’ substance abuse. The law is codified at 42 U.S.C. §290dd-2. The implementing Federal regulations, “Confidentiality of Alcohol and Drug Abuse Patient Records,” are contained in 42 CFR Part 2 (Vol. 42 of the Code of Federal Regulations, Part 2).
Federal law and regulations severely restrict communications about identifiable patients by “programs” providing substance use diagnosis, treatment, or referral for treatment (42 CFR §2.11). The purpose of the law and regulations is to decrease the risk that information about individuals in recovery will be disseminated and that they will be subjected to discrimination, which should also encourage people to seek treatment for substance use disorders.
In most primary care settings, Federal confidentiality laws and regulations do not apply. For many years, there was confusion about whether general medical care settings such as primary care clinics or hospital emergency rooms were subject to Federal law and regulations because they provided substance abuse diagnosis, referral, and treatment as part of their services. In 1995, DHHS revised the definition of the kinds of “programs” subject to the regulations, making it clear that the regulations do not usually apply to a general medical care facility unless that facility (or person) “holds itself out as providing, and provides, alcohol or drug abuse diagnosis, treatment or referral for treatment” (42 CFR §2.11).
Most primary care clinicians are not subject to Federal rules. Practitioners should be aware, however, that if a health care practice includes someone whose primary function is to provide substance abuse assessment or treatment and if the practice benefits from “Federal assistance,” that practice must comply with the Federal law and regulations and implement special rules for handling information about patients who may have substance abuse problems.
Moreover, the fact that most primary care clinicians are not subject to the Federal rules does not mean that they can handle information about patients’ substance use problems in a cavalier manner. Because of the potential for damage to patients, clinicians should always handle such information with great care or the professional licensing sections or both. The State licensing authority as well as professional associations can usually help answer such questions.
It is essential that all EtG testing is governed by HIPAA privacy laws and that patient information is stored and transmitted within the governance of these laws and a Chain of Custody.
SmarTest Labs located in the Maryland area, provides EtG testing for individuals, organizations, and academic and clinical organizations. We use state-of-the-art technologies (Chain of Custody and HIPAA controls). Depending on the circumstances our approach customizes the appropriate test for you (Hair, Nail, Blood, Breadth, or Urine). Contact Smartest Labs for your next EtG test.
Recent Comments